
The U.S. Food and Drug Administration (FDA) has recently granted approval to Daxxify, a novel injected medicine aimed at reducing facial wrinkles in adults. Dermatologists consider this approval a significant milestone as it introduces the first major competitor to Botox in decades. Daxxify belongs to the class of neuromodulators, which includes well-known drugs like Botox, Dysport, Xeomin, and Jeuveau. However, what sets Daxxify apart is its potential to provide longer-lasting results compared to its counterparts.
Both Daxxify and Botox fall under the category of neuromodulators, working by injecting small and precise amounts of botulinum toxin into underlying muscles. This process causes muscle relaxation, smoothes the overlying skin, and temporarily reduces the appearance of wrinkles. The only difference is that Daxxify seems to take effect faster than Botox and Dysport. Daxxify seems to set in within a day or two while Dysport takes 3 to 5 days and Botox takes 4 to 7 days to start seeing results.
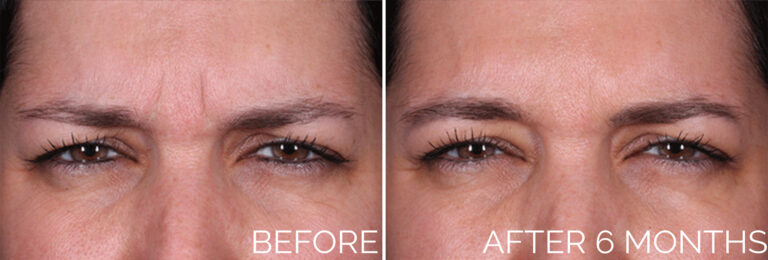
Clinical trials submitted for FDA approval demonstrated that Daxxify may offer a longer duration of effectiveness. Approximately 80 percent of users exhibited little or no facial wrinkles after four months, with about half maintaining minimal wrinkles even after six months, while Botox users show signs of wrinkles after 3 months.
Unlike Botox and some other neuromodulators made from human serum albumin, Daxxify is formulated using peptides or amino acids. This composition, along with its lack of human or animal products, may contribute to its potentially extended wrinkle-smoothing effects.
The FDA approved Daxxify based on clinical trials involving over 2,700 patients who received 4,200 treatments. One month after the injections, 98 percent of patients exhibited little or no wrinkles. Even after six months, half of the patients were still nearly wrinkle-free, and some reported results lasting up to nine months.
In clinical trials, Daxxify demonstrated minimal treatment-related side effects. Most commonly reported were headaches with 6% of users, drooping eyelids with 2% percent of users, and facial asymmetry with 1% of users..
Although Daxxify is currently approved only for smoothing frown lines, similar to Botox, dermatologists anticipate potential off-label usage for other facial areas and medical conditions, as has been seen with Botox.
Daxxify’s extended longevity may make it more suitable for individuals who have already tried other injectable treatments. Dermatologists recommend starting with temporary options before committing to longer-lasting products.
As of now, pricing information for Daxxify has not been disclosed by Revance, the company that manufactures the drug. Dermatologists expect Daxxify to be widely available for patients in the United States sometime in 2023.
Daxxify’s approval marks an exciting development in the realm of facial wrinkle treatments, offering a potential alternative to established neuromodulators like Botox and Dysport. Its longevity and peptide-based formulation have captured the attention of dermatologists, who are eagerly anticipating its market availability and potential benefits for patients seeking a longer-lasting solution for wrinkle reduction.